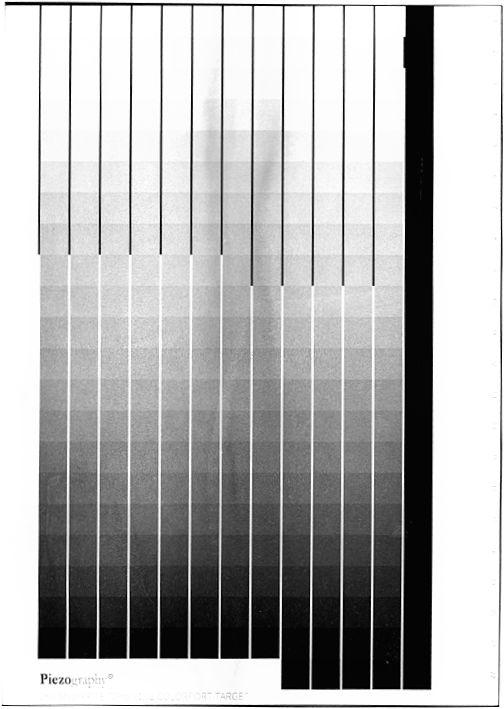
My recent Pd/Pt prints have been muddy and lacked clear highlights. I’ve spent the past three weeks trying to get to the bottom of my fog problem.
As a test, I processed a fresh sheet of sensitized (50%FO/50%Pd/Pt) Hahnemuhle Platinum Rag paper, with absolutely no UV exposure. It turned ~30% grey immediately after development. The grey would stubbornly not change at all despite long soaks in three EDTA clearing baths. So, I further dimmed my LED lights, mixed new Ferric Oxalate, switched from brush to glass rod application, replaced the Ammonium Citrate developer, and all three EDTA clearing baths.
Still, an unexposed test strip turned muddy grey showing significant variegation.
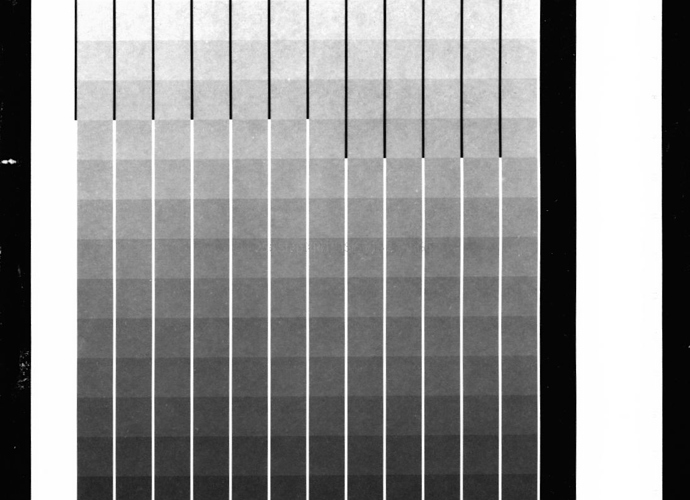
Finally, as a last resort, I broke open a different, more recent package of 11x15" HPR and made a few new test strips. Voilá! The paper cleared instantly of all grey tones and yellow stain immediately. Bright white paper! I was gleeful, but, still perplexed.
How can the ostensibly same paper vary so much from batch to batch so as to produce such differing results when all other factors of chemistry, temperature, pH, and humidity, are the same? Does paper deteriorate as a support for Pd/Pt printing over time or exposure to air, heat, and humidity? Or, is the difference in the manufacturing process that somehow varies?
Any info you may have on this topic would help me, and perhaps others, avoid similar setbacks such in the future.
(Attached are two test prints in which all exposure and processing factors are the same. The only difference is that in the lighter cleared print, the paper is from a different package.)